[ベスト] favipiravir 600mg 687005-Favipiravir 200mg
Regulatory Information LicNo /147 Country of Registration TURKEY Virtual Innovation Day Simulation study showed that the 1600 mg/600 mg BID regimen was insufficient for the treatment of COVID19 targeting EC50 (97µg/mL), especially in patients with larger BSA and/or IMV A higher favipiravir dosage is required for COVID19, but dosedependent nonlinear pharmacokinetics may cause an unexpected significant pharmacokinetic change and drug toxicity Favipiravir, previously known as T705, is a prodrug of the purine nucleotide favipiravir ribofuranosyl5′triphosphate The active agent
Glenmark Pharma Drops Price Of Covid 19 Drug Favipiravir To Rs 75 Tablet Latest News India Hindustan Times
Favipiravir 200mg
Favipiravir 200mg-Ha az orvos másként nem rendeli, a kezelés első napján 2×8 db tabletta (2×1600 mg) bevétele javasolt Ezt követően a kezelést napi 2×3 db tabletta (2×600 mg) szedésével javasolt folytatniFluimucil 600mg Eff Rp Beli FLUIMUCIL 600MG EFF di K24klik, 100% asli dan dapatkan manfaatnya sebagai obat pengencer dahak pada kondisi bronkit




Influenza Drug Favipiravir Is Being Tested To Treat Covid 19 Clinical Trials Arena
Beli Fluimucil 600 terlengkap harga murah July 21 di Tokopedia! The dosing regimen is an important part of successful antiviral therapy The approved favipiravir dosing regimen for influenza in Japan is a loading dose of 30 mg on day 1, followed by a maintenance dose of 600 mg twice daily on days 2–5 (Furuta et al, 17, Wang et al, a, Wang et al, b)Favipiravir Observational Study Interim Report 3 Details Retrospective analysis of favipiravir use in 10,986 hospitalized patients, including analysis of changes in clinical status and side effects Common adverse events were uric acid level increase and liver function enzyme increase Authors
Dosing Adult Note Favipiravir is currently under investigation for use in the treatment of coronavirus disease 19 (COVID19) (See ClinicalTrialsgov)At this time, safety and efficacy data are limited However, preliminary dosing information based on the available published evidence and ongoing clinical trials is provided Sample size 15 or 30 severe influenza patients Research hypothesis The administration of oral favipiravir at either 1600mg/600mg BID or 1800/800mg BID will result in ≥ 80% patients achieving a minimum observed plasma trough concentration above the MEC (μg/ml) at all measured time points after the second dose Sun Pharma latest to sell COVID19 drug favipiravir in India, to cost Rs 35 per tablet 04 Aug, , 0410 PM IST Favipiravir, along with another antiviral, remdesivir, has emerged as one of the most soughtafter drugs at hospitals fighting COVID19 in India, which saw a surge of 50,000plus infections for the sixth straight day on Tuesday
Favipiravir tablets should be swallowed whole with water The tablet should not be opened, broken, or chewed For patients with COVID19, the usual dosage of favipiravir for adults is 1800 mg orally twice daily on day 1, followed by 800 mg orally twice daily for 14 daysThe usual adult dosage is 1600 mg of Favipiravir administered orally twice daily on Day 1, followed by 600 mg orally twice daily from Day 2 to Day 5 or as directed by physicians The total treatment duration should be 5 days Group 2 included 50 patients, who received the investigational drug favipiravir in a regimen of 30 mg (1600 mg 12 hourly) loading dose on Day 1 followed by 10 mg maintenance dose (600 mg 12




Favipiravir An Anti Influenza Drug Against Life Threatening Rna Virus Infections Sciencedirect




Sunpharma Launches Favipiravir Tablet Fluguard To Treat Mild To Moderate Covid 19 Infections At Rs 35 Per Pill Technology News Firstpost
Favipiravir is a prodrug that is ribosylated and phosphorylated intracellularly to form the active metabolite Favipiravir ribofuranosyl5′triphosphate (Favipiravir RTP) After an oral administration in adults at 1600 mg twice on day 1, then 600 mg twice daily (1600 mg/600 mg BID); For adults, provide oral administration of 1600mg of favipiravir twice a day on Day 1, and 600mg of favipiravir twice a day from Day 2 to Day 5 The total administration period is five days Date of manufacturing and sales approvalOn day 1 and day 6 the estimated AUC was and 553




Phase 2a Open Label Dose Escalating Multi Center Pharmacokinetic Study Of Favipiravir T 705 In Combination With Oseltamivir In Patients With Severe Influenza Sciencedirect




Faravir Favipiravir 0 Mg Tablets Inozed Linezolid Tablets 600 Mg Manufacturer From Hyderabad
Favipiravir (1600mg*2/first day followed by 600mg*2/day) for 10 days The primary outcome was clinical recovery rate of Day 7 Latency to relief for pyrexia and cough, the rate of auxiliary oxygen therapy (AOT) or noninvasive mechanical ventilation (NMV) were the secondary outcomes Safety data were collected for 17 daysHighdose favipiravir (10 mg favipiravir BID for 1 day, followed by 800 mg favipiravir BID for 4 days) PV Pharma is Favipiravir Tablet Manufacturer and Suppliers in India and Favipiravir Tablet 0mg, 400mg, 600mg Exporter in India




Covid 19 Living Data



Eunethta Eu Wp Content Uploads 08 Eunethta Covid 19 Rcr11 Favipiravir August Final Pdf
Enrolled patients will be randomly assigned to 1 of 3 parallel treatment dose groupsPlacebo;= 10) Favipiravir 600 mg tid with 1,600 mg first loading dosage, no more than 14 days Group C ( = 10) LopinavirRitonavir 0 mg/50 mg, twice daily, for 14 days 1 Time to viral negativity by RTPCR 2 Time to clinical improvement Time from start of study drug to hospital discharge or to NEWS < 2 for 24 hours ChiCTRFavipiravir, sold under the brand name Avigan among others, is an antiviral medication used to treat influenza in Japan It is also being studied to treat a number of other viral infections, including SARSCoV2 Like the experimental antiviral drugs T1105 and T1106, it is a pyrazinecarboxamide derivative It is being developed and manufactured by Toyama Chemical (a subsidiary of Fujifilm




Favipiravir And The Need For Early Ambulatory Treatment Of Sars Cov 2 Infection Covid 19 Antimicrobial Agents And Chemotherapy




Early Onset Favipiravir Saves Lives Research Square
The 'BDFAVI' 400 mg tablets will be priced at ₹990 for a strip of 10 tablets, BDR Pharma said in a statement Each tablet will cost ₹99 Drugscom provides accurate and independent information on more than 24,000 prescription drugs, overthecounter medicines and natural products This material is provided for educational purposes only and is not intended for medical advice, diagnosis or treatment Data sources include IBM Watson Micromedex (updated 1 July 21), Cerner Multum™ (updated 1Lowdose favipiravir (1000 mg favipiravir BID for 1 day, followed by 400 mg favipiravir BID for 4 days);




Glenmark Launches New Coronavirus Medicine At 103 Per Tablet 10 Things To Know




Glenmark Pharma Drops Price Of Covid 19 Drug Favipiravir To Rs 75 Tablet Latest News India Hindustan Times
Methods We conducted a prospective, randomized, controlled, openlabel multicenter trial involving adult patients with COVID19 Patients were randomly assigned in a 11 ratio to receive conventional therapy plus Umifenovir (Arbidol) (0mg*3/day) or Favipiravir (1600mg*2/first day followed by 600mg*2/day) for 10 daysThe approved dosage of favipiravir is "1600 mg orally twice daily for 1 day followed by 600 mg orally twice daily for 4 days"Jolly Healthcare Offering Favipiravir 0mg Tablet, Glenmark Pharmaceuticals, 5*10 Tabs at Rs 1030/strip in Jaipur, Rajasthan Read about company Get contact details and address




Favipiravir Versus Arbidol For Covid 19 A Randomized Clinical Trial Medrxiv



Www European Virus Archive Com Sites Default Files Covid19 Compounds Tests Favipiravir Pdf
Blood Concentrations The following table shows pharmacokinetic parameters of favipiravir after an oral administration in 8 healthy adults at 1600 mg twice daily for 1 day, then 600 mg twice daily for 4 days followed by 600 mg once daily for 1 day (1600 mg/600 mg BID)Favipiravir, an antiviral drug that has been used in the treatment of influenza in Japan since 14, was approved by the Drug Controller General of India for treatment of mild to moderate Covid In the clinical study conducted by the National Clinical Research Center for Infectious Diseases in Shenzhen, two 1,600mg doses of favipiravir on the first day and two doses of 600mg for the following 13 days, in



Favipiravir Tablet Suppliers In India Favipiravir Tablet Exporter 0mg 400mg 600mg Exporter In India Pv Pharma



海正药业
Favipiravir, sold under the brand name Avigan, is an antiviral medication used to treat influenza in Japan It is also being studied to treat a number of other viral infections Like the experimental antiviral drugs (T1105 and T1106), it is a pyrazinecarboxamide derivativeThe usual dosage of favipiravir for adults is 1600 mg orally twice daily for 1 day followed by 600 mg orally twice daily for 4 days The total administration period should be 5 days PRECAUTIONS 1 Careful Administration (AVIGAN should be administered with care in the following patients) Patients with gout or a history of gout, and patients withIn this trial, on day 1 dose of 1600 mg twicedaily favipiravir was used and on days 214 favipiravir dose was 600 mg twicedaily On contrast in same study 400 mg lopinavir and 100 mg ritonavir twicedaily was the dose of lopinavirritonavir Both favipiravir and lopinavirritonavir were continued until 14 days had passed or until viral



Frontiers Drugs For Influenza Treatment Is There Significant News Medicine



Heatinformatics Com Sites Default Files Images Videosfilecontent jve 6 2 06 Hill Rev3 Pdf
Favipiravir sold under the brand of Covihalt Tablet from Lupin Pharma is an Antiviral medicine Favipiravir is used to treat mild cases of COVID19 The drug has found to be effective in suppressing the growth of virus and helping in faster recovery of patients Prescription requiredDosage & Administration The usual adult dosage is 1600 mg of Favipiravir administered orally twice daily on Day 1, followed by 600 mg orally twice daily from Day 2 to Day 5 or as directed by physicians The total treatment duration should be 5 days Conventional therapy plus umifenovir (Arbidol) (0 mg thrice a day) or favipiravir (1600 mg twice daily followed by 600 mg twice daily) for 7 days (extendable to 10 days) The study comprised 240 patients with 11 randomization to both groups



Favipiravir For The Treatment Of Patients With Covid 19 A Systematic Review And Meta Analysis Bmc Infectious Diseases Full Text



Favipiravir Tablet Suppliers In India Favipiravir Tablet Exporter 0mg 400mg 600mg Exporter In India Pv Pharma
Blood Concentrations The following table shows pharmacokinetic parameters of favipiravir after an oral administration in 8 healthy adults at 1600 mg twice daily for 1 day, then 600 mg twice daily for 4 days followed by 600 mg once daily for1 day (1600 mg / 600 mg BID)FAVIPIRAVIR KF 0MG FC TAB 100S termasuk obat antivirus yang digunakan untuk mengatasi infeksi akibat virus influenza Konsultasikan terlebih dahulu kepada dokter apabila akan digunakan pada pasien dengan riwayat atau kondisi sakit seperti Riwayat alergi terhadap kandungan obat ini Riwayat penyakit ginjal∙ Promo Pengguna Baru ∙ Kurir Instan ∙ Bebas Ongkir ∙ Cicilan 0%




Favipiravir Antiviral Efficacy Against Sars Cov 2 In A Hamster Model Biorxiv



Www Pmda Go Jp Files Pdf
For influenza treatment, patients are given a 1600 mg dose of this drug on Day 1 and about 600 mg dose from day 2 to day 5 The flexibility that favipiravir shows in its ability to act on multiple different influenza viruses has led to other countries using the drug to explore treatments for novel (emerging) viruses including Ebola and mostThe reported CL/F for favipiravir 1600 mg dosed once daily is 298 L/hr ±030 and the CL/F values for favipiravir 600 mg dosed twice daily on days 12 and once daily on days 37 were 672 L/hr ±168 on Day 1, and 2 L/hr ±091 on Day 7 There is currently no reported clearance data for favipiravir 1600 mg dosed twice dailyA készítmény ajánlott adagja A Favipiravir Egis adagját kezelőorvosa állapítja meg, orvosa utasításait maradéktalanul tartsa be!



Apps Who Int Iris Bitstream Handle Who 19 Ncov Remdesivir 1 Eng Pdf




Influenza Drug Favipiravir Being Tested To Treat Covid 19
In this clinical trial, researchers treated 35 patients of COVID19 pneumonia with Favipiravir (30 mg on the first day, 10 mg/day on the 2nd to 14th day, 600mg by twice daily and the virus was eliminated within 14 days)Molecule Description Favipiravir is an antiviral medicine used to treat mild to moderate COVID19 disease under emergency conditions It works by inhibiting the replication of infection causing virus Before using this m edicine, it is advised to inform the doctor about your detailed medical and medication history Antivirus Favipiravir (AviganIndofarma) 600mg 2x sehari selama 5 hari 3 Anti



Sidp Org Resources Documents Covid19 Daniel chastain 4 24 handouts Pdf




Favipiravir At High Doses Has Potent Antiviral Activity In Sars Cov 2 Infected Hamsters Whereas Hydroxychloroquine Lacks Activity Pnas
It has recently been demonstrated that, as a prodrug, Favipiravir (half maximal effective concentration (EC50) = 61 μmol·L1, halfmaximal cytotoxic concentration (CC50) > 400 μmol·L1, selectivity index (SI) > 646) effectively inhibits the SARSCoV2 infection in Vero E6 cells (ATCC1586) Dosis dan Aturan Pakai Favipiravir Menurut penelitian yang sedang dilakukan, favipiravir diberikan dalam dosis 1600 mg sebanyak 2 kali sehari pada hari pertama, dilanjutkan dengan 600 mg sebanyak 2 kali sehari pada hari ke2 hingga hari ke5 Penggunaan favipiravir dalam penanganan infeksi virus Corona akan dipertimbangkan oleh dokter sesuai The patients were given Favipiravir tablets 3,600mg on day one (1,800mg twice daily) and 1,600mg (800mg twice daily) on day two or later for up to a maximum of 14 days Data showed that the drug provided multiple treatment benefits including faster time to clinical cure and even delayed the need for supportive oxygen therapy




Phase 2a Open Label Dose Escalating Multi Center Pharmacokinetic Study Of Favipiravir T 705 In Combination With Oseltamivir In Patients With Severe Influenza Ebiomedicine



Safety And Efficacy Of Favipiravir Versus Hydroxychloroquine In Management Of Covid 19 A Randomised Controlled Trial Scientific Reports




Favipiravir At High Doses Has Potent Antiviral Activity In Sars Cov 2 Infected Hamsters Whereas Hydroxychloroquine Lacks Activity Pnas
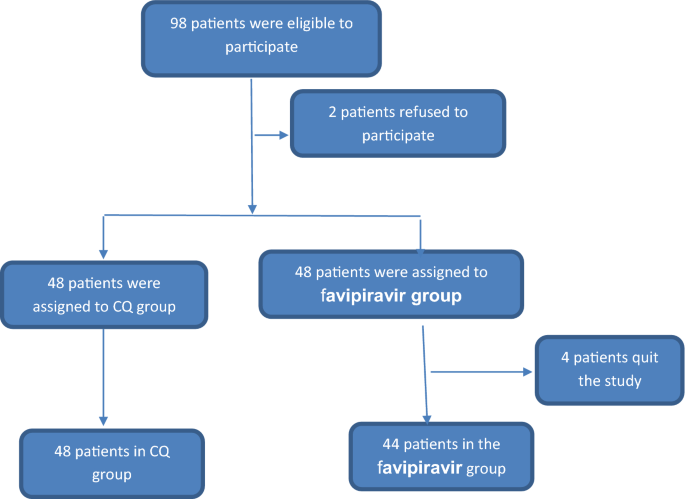



Efficacy Of Favipiravir In Covid 19 Treatment A Multi Center Randomized Study Springerlink




Nipro Jmi Pharma Ltd First Japanese Joint Venture Pharmaceuticals In Bangladesh




Phase 2a Open Label Dose Escalating Multi Center Pharmacokinetic Study Of Favipiravir T 705 In Combination With Oseltamivir In Patients With Severe Influenza Ebiomedicine



Www Nature Com Articles S 021 6 Pdf Origin Ppub



Everything You Need To Know About Favipiravir The Potential Drug For Covid 19 Treatment Health News Firstpost



A Multicenter Non Randomized Uncontrolled Single Arm Trial For Evaluation Of The Efficacy And The Safety Of The Treatment With Favipiravir For Patients With Severe Fever With Thrombocytopenia Syndrome




Converge Biotech Announces The Launch Of Vergiflu Favipiravir 0 Mg In India To Treat Mild To Moderate Covid 19 Hindustan Times



Www Kansensho Or Jp Uploads Files Topics 19ncov Covid19 Casereport En 0512 Pdf




The Japanese Government Delivered 120 Avigan Tablets For Treatment Of Covid 19 In Indonesia Portal Kementerian Luar Negeri Republik Indonesia
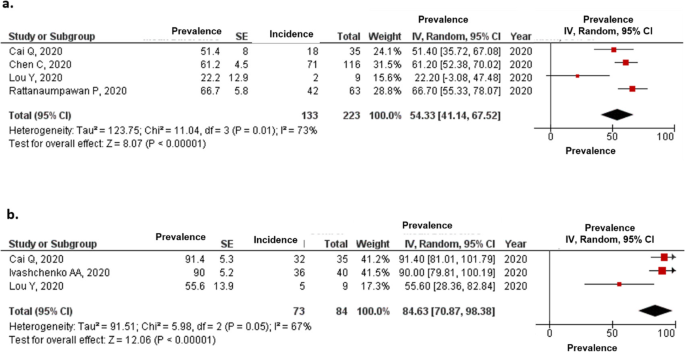



Favipiravir For The Treatment Of Patients With Covid 19 A Systematic Review And Meta Analysis Bmc Infectious Diseases Full Text



A Multicenter Non Randomized Uncontrolled Single Arm Trial For Evaluation Of The Efficacy And The Safety Of The Treatment With Favipiravir For Patients With Severe Fever With Thrombocytopenia Syndrome




Favipiravir Pharmacokinetics And Concerns About Clinical Trials For 19 Ncov Infection Du Clinical Pharmacology Amp Therapeutics Wiley Online Library




Halodoc




Systematic Review On The Therapeutic Options For Covid 19 Clinical Ev Idr



Investigational Treatments For Covid 19 The Pharmaceutical Journal
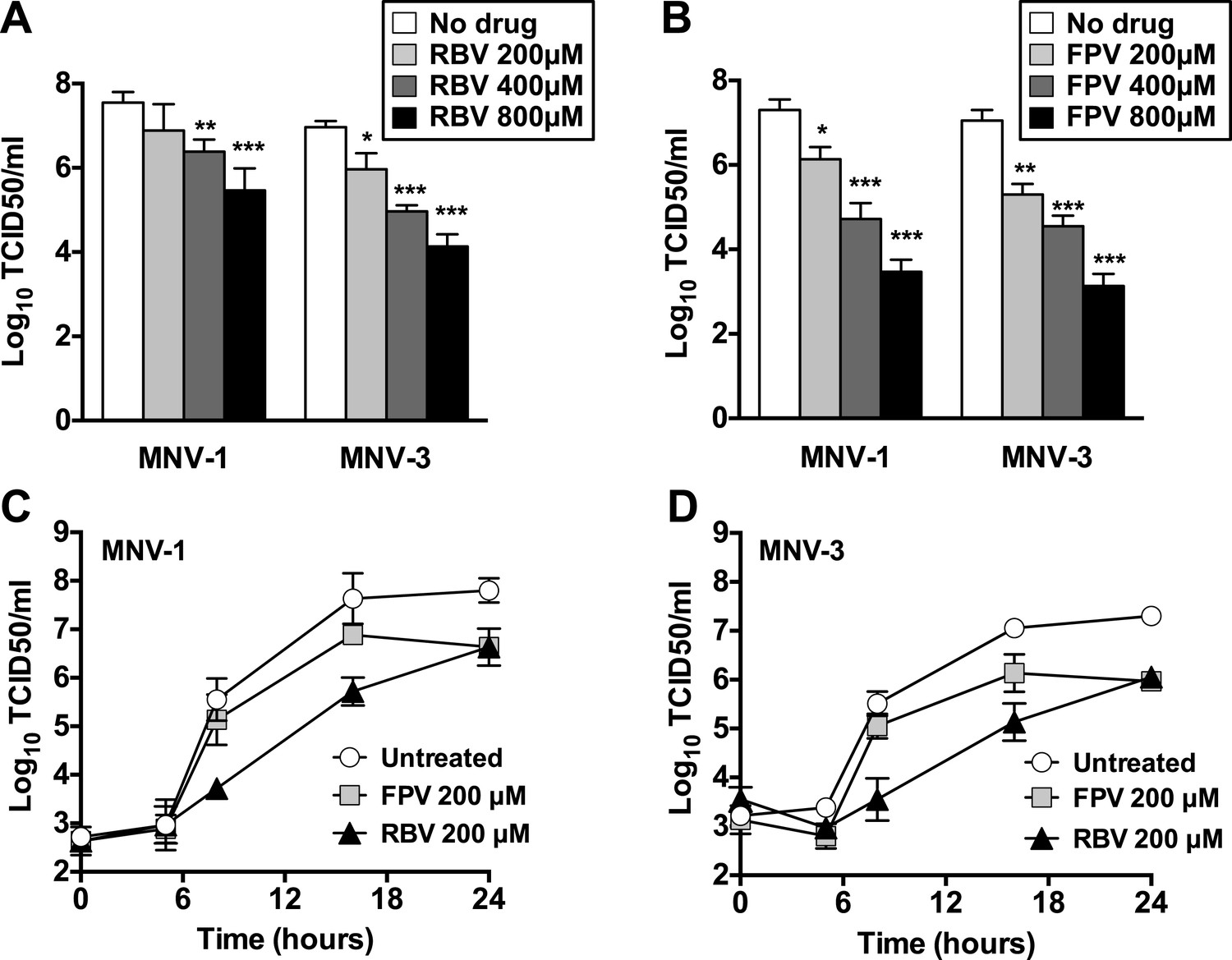



Favipiravir Elicits Antiviral Mutagenesis During Virus Replication In Vivo Elife




Favipiravir A New And Emerging Antiviral Option In Covid 19 Sciencedirect



Www Ijidonline Com Article S11 9712 6 Pdf



Sidp Org Resources Documents Covid19 Daniel chastain 4 24 handouts Pdf




Covid 19 Pneumonia In Kidney Transplant Recipients A Promising Treatment Algorithm In The Absence Of A Disease Specific Drug Karatas Journal Of Medical Virology Wiley Online Library




Influenza Drug Favipiravir Is Being Tested To Treat Covid 19 Clinical Trials Arena
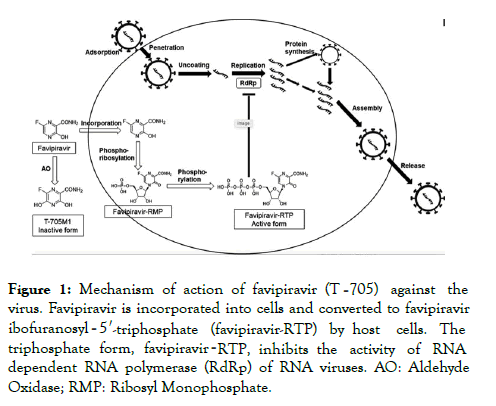



Favipiravir Promising Therapy For Covid 19




Fabi Flu Glenmark 5 Points About The Drug Approved For Covid 19 Treatment In India
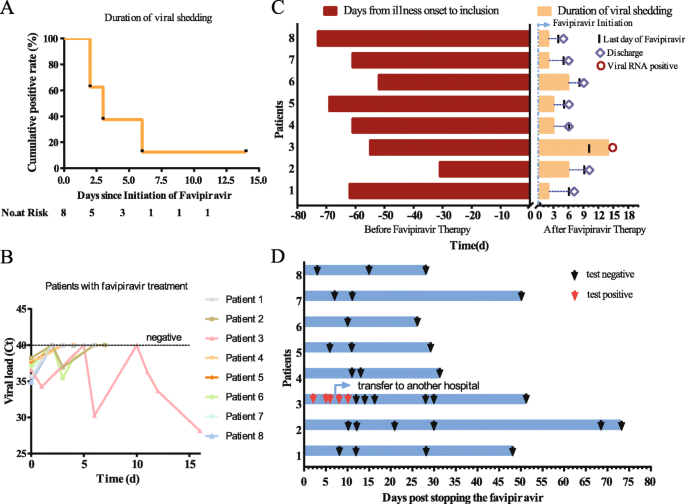



Oral Favipiravir For Patients With Delayed Sars Cov 2 Viral Rna Clearance A Case Series Critical Care Full Text




Favipiravir Antiviral Efficacy Against Sars Cov 2 In A Hamster Model Biorxiv



Favipiravir Priced Lower In India Than In Other Countries Says Glenmark The Hindu Businessline
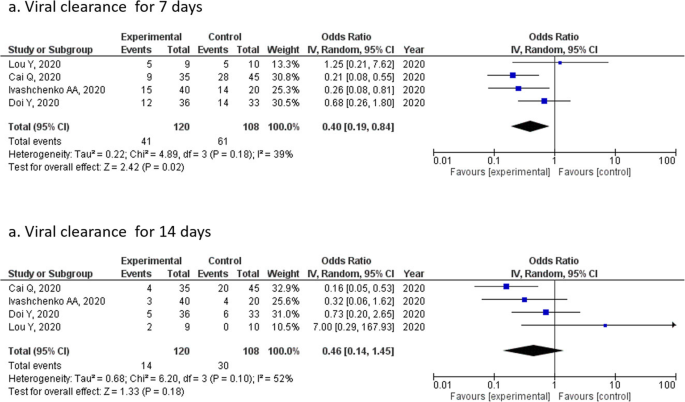



Favipiravir For The Treatment Of Patients With Covid 19 A Systematic Review And Meta Analysis Bmc Infectious Diseases Full Text




Pharmaceutical Tablet Fabiflu Favipiravir 0 Mg Tablets Exporter From Delhi



Ascpt Onlinelibrary Wiley Com Doi Pdf 10 1002 Cpt 1844




Favipiravir A New Medication For The Ebola Virus Disease Pandemic Disaster Medicine And Public Health Preparedness Cambridge Core
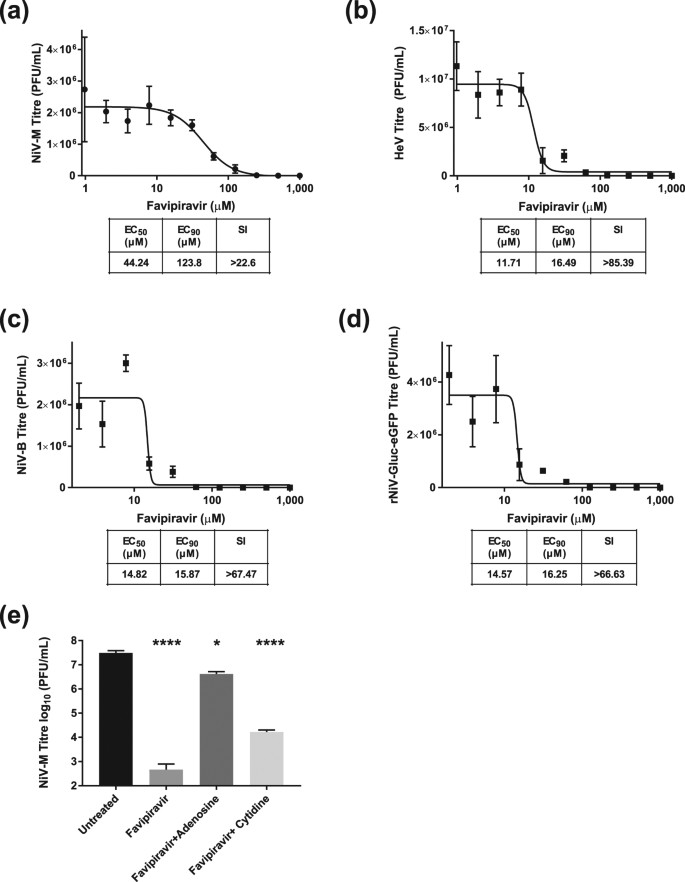



Favipiravir T 705 Protects Against Nipah Virus Infection In The Hamster Model Scientific Reports




Minimum Costs To Manufacture New Treatments For Covid 19 Sciencedirect
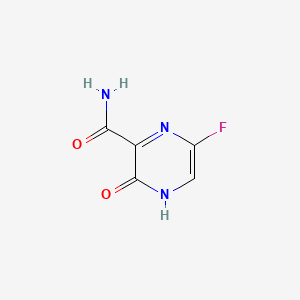



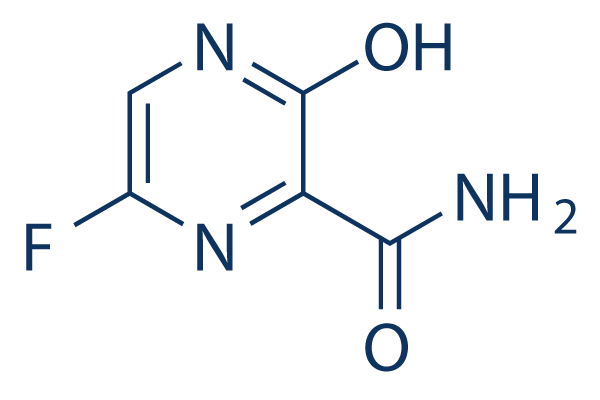
Favipiravir C5h4fn3o2 Pubchem




Role Of Favipiravir In The Treatment Of Covid 19 International Journal Of Infectious Diseases




A Prospective Randomized Open Label Trial Of Early Versus Late Favipiravir Therapy In Hospitalized Patients With Covid 19 Antimicrobial Agents And Chemotherapy




Pdf Adjunctive Favipiravir For Severe Covid 19 A Retrospective Observational Study Of The First 41 Patients In Thailand




Cdei Clinical Outcomes And Plasma Concentrations Of Baloxavir Marboxil And Favipiravir In Covid 19 Patients An Exploratory Randomized Controlled Trial Cdei




Hetero Launches Generic Covid 19 Drug Favivir At Rs 59 Per Tablet Business Standard News



Mji Ui Ac Id Journal Index Php Mji Article Download 4652 1936



Www Psmid Org Wp Content Uploads 21 04 Treatment Favipiravir Pdf




Experimental Treatment With Favipiravir For Covid 19 An Open Label Control Study Sciencedirect




Pathophysiological Basis And Rationale For Early Outpatient Treatment Of Sars Cov 2 Covid 19 Infection The American Journal Of Medicine




Characteristics Of 4 Critically Ill Patients Who Received Favipiravir Download Scientific Diagram



Cdei Favipiravir Avigan Vs Umifenovir Arbidol For Covid 19 Cdei




Jual Obat Avigan Tablet Farmaku




Favipiravir Versus Arbidol For Covid 19 A Randomized Clinical Trial Medrxiv



Fabiflu Is The Most Economical Covid 19 Treatment Option Glenmark S Reply To Centre On Alleged Overpricing




Intravenous Immunoglobulin And Favipiravir Treatment For A Kidney Transplant Patient With Severe Covid 19 Pneumonia Transfusion And Apheresis Science




Favipiravir Pharmacokinetics And Concerns About Clinical Trials For 19 Ncov Infection Du Clinical Pharmacology Amp Therapeutics Wiley Online Library



Www Who Int Blueprint Priority Diseases Key Action Rdblueprintbtxexpertgrouponfavipiravircallapril10th Pdf




Favipiravir Elicits Antiviral Mutagenesis During Virus Replication In Vivo Elife
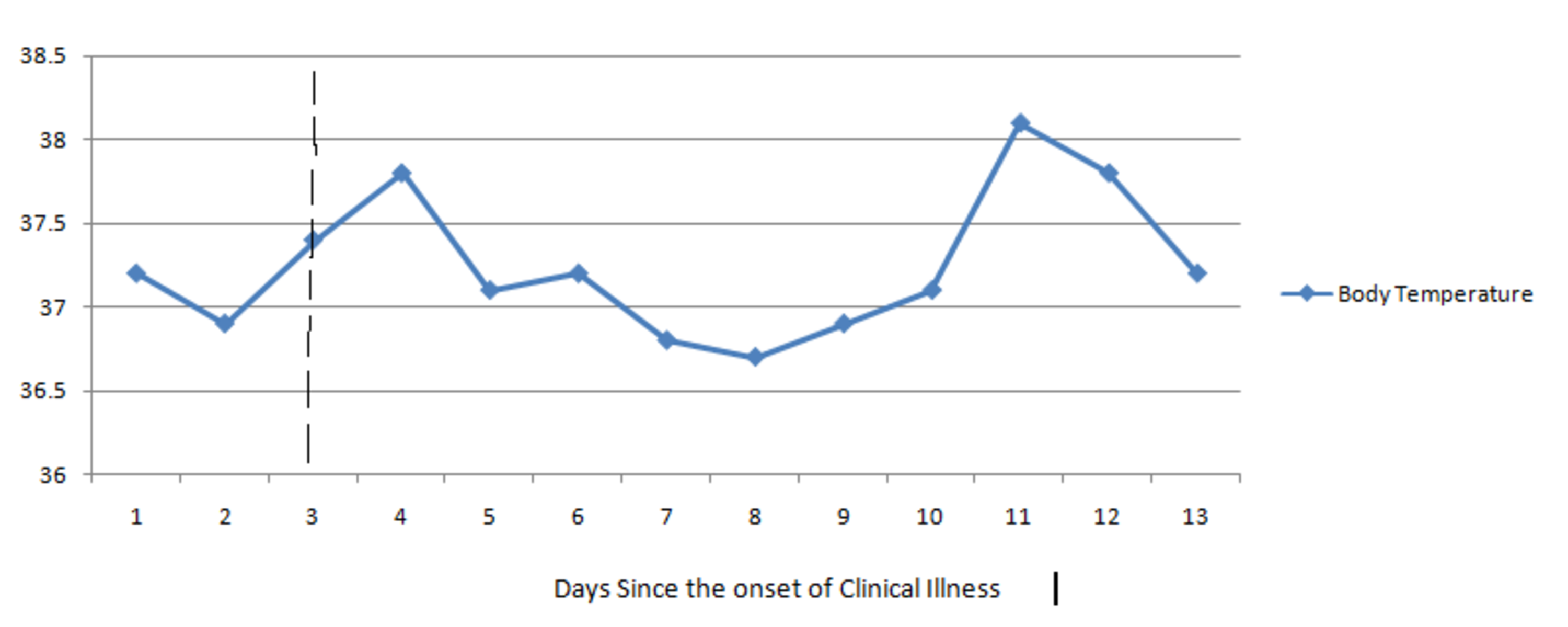



Favipiravir Induced Drug Fever In A Young Adult Covid 19 Patient Cureus




Covid 19 Living Data



Www Sukl Cz File 929 1 1




Favipiravir At High Doses Has Potent Antiviral Activity In Sars Cov 2 Infected Hamsters Whereas Hydroxychloroquine Lacks Activity Pnas



Favipiravir T 705 99 Hplc Selleck Dna Rna Synthesis Inhibitor
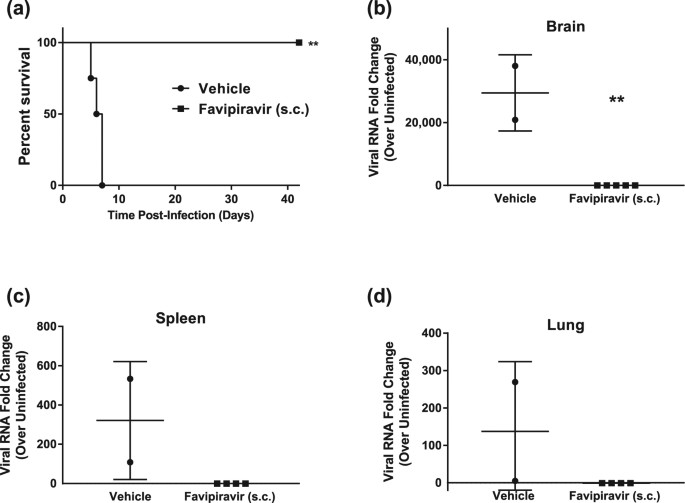



Favipiravir T 705 Protects Against Nipah Virus Infection In The Hamster Model Scientific Reports




Favipiravir At High Doses Has Potent Antiviral Activity In Sars Cov 2 Infected Hamsters Whereas Hydroxychloroquine Lacks Activity Pnas
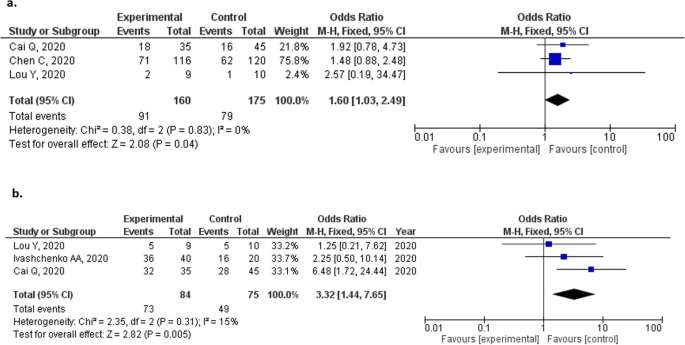



Favipiravir For The Treatment Of Patients With Covid 19 A Systematic Review And Meta Analysis Bmc Infectious Diseases Full Text
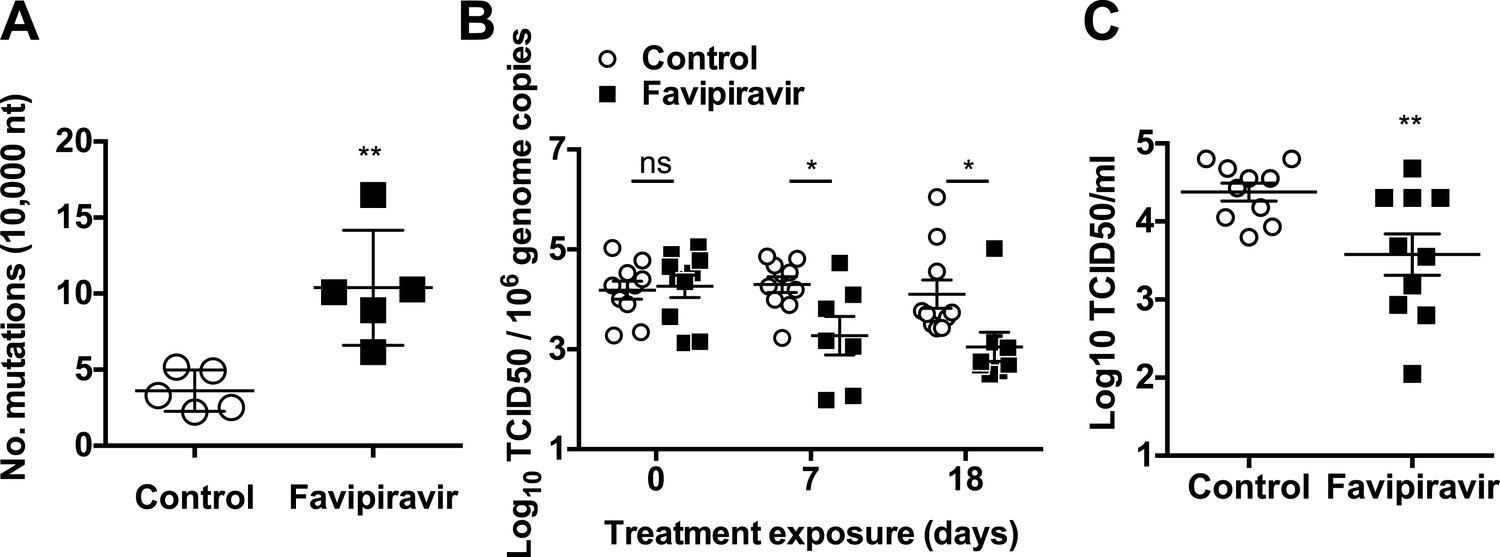



Favipiravir Elicits Antiviral Mutagenesis During Virus Replication In Vivo Elife




Favipiravir T 705 A Broad Spectrum Inhibitor Of Viral Rna Polymerase Abstract Europe Pmc
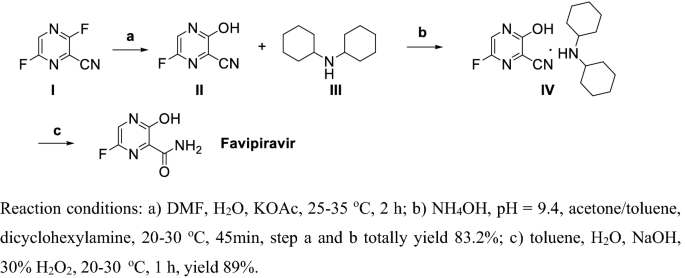



Reviews On Biological Activity Clinical Trial And Synthesis Progress Of Small Molecules For The Treatment Of Covid 19 Springerlink



Ir Fujifilm Com En Investors Ir Materials Disclosure Materials Backnumber Disclosure Materials Main 0 Link Ff Announcement 001 Pdf



Pharmacological Effects Of Favipiravir On Coronavirus An Update Biomedical And Pharmacology Journal
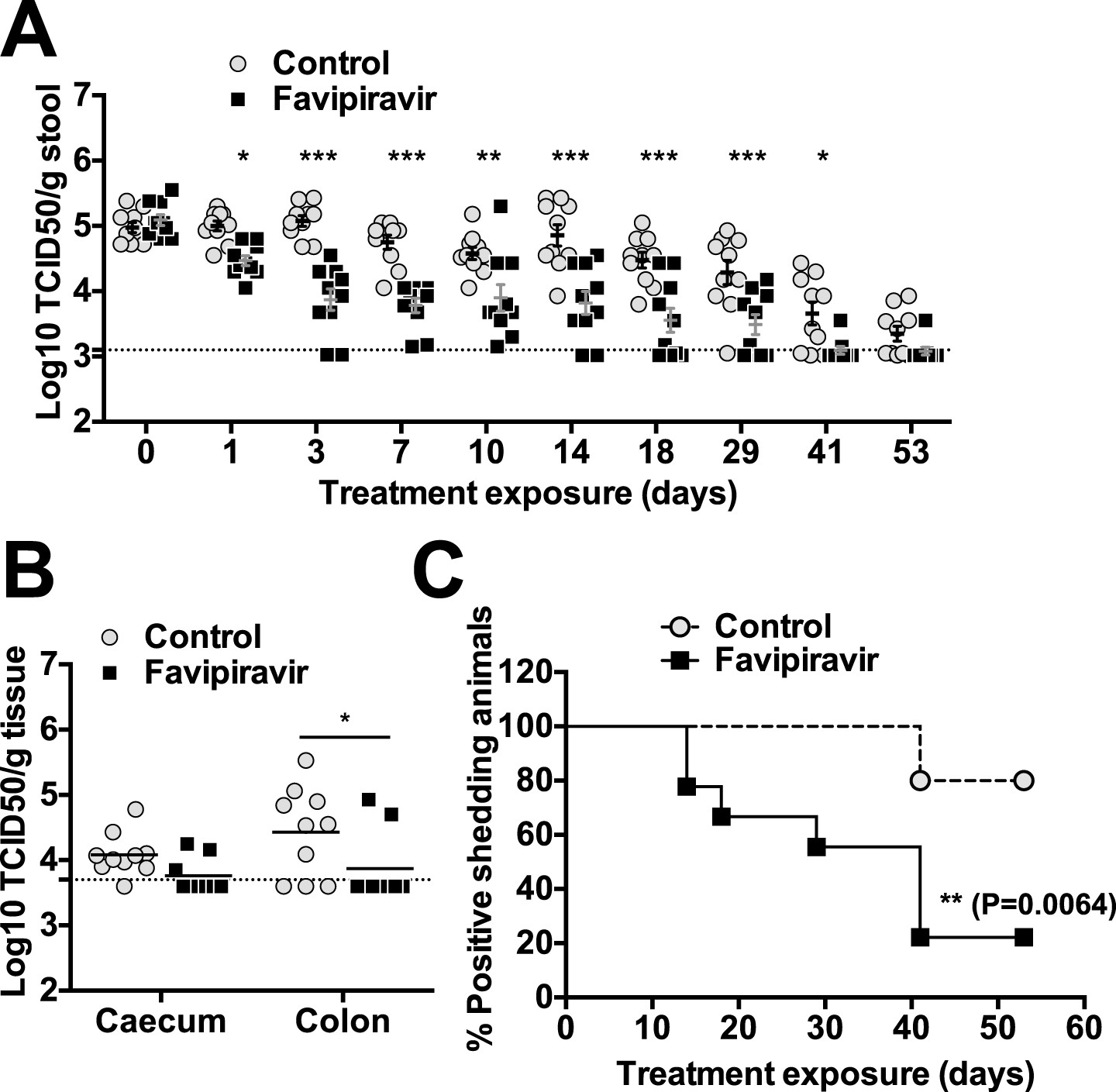



Favipiravir Elicits Antiviral Mutagenesis During Virus Replication In Vivo Elife



Www Psmid Org Wp Content Uploads 04 Favipiravir Abridged 4apr Ver3 Pdf



Www Pnas Org Content Pnas Early 10 08 Full Pdf




A Comparative Analysis Of Remdesivir And Other Repurposed Antivirals Against Sars Cov 2 Embo Molecular Medicine




Favipiravir Versus Other Antiviral Or Standard Of Care For Covid 19 Treatment A Rapid Systematic Review And Meta Analysis Research Square



Www Cdc Gov Tw File Get Ht8juib Mi Aknlwstwzvw




Phase 2a Open Label Dose Escalating Multi Center Pharmacokinetic Study Of Favipiravir T 705 In Combination With Oseltamivir In Patients With Severe Influenza Ebiomedicine
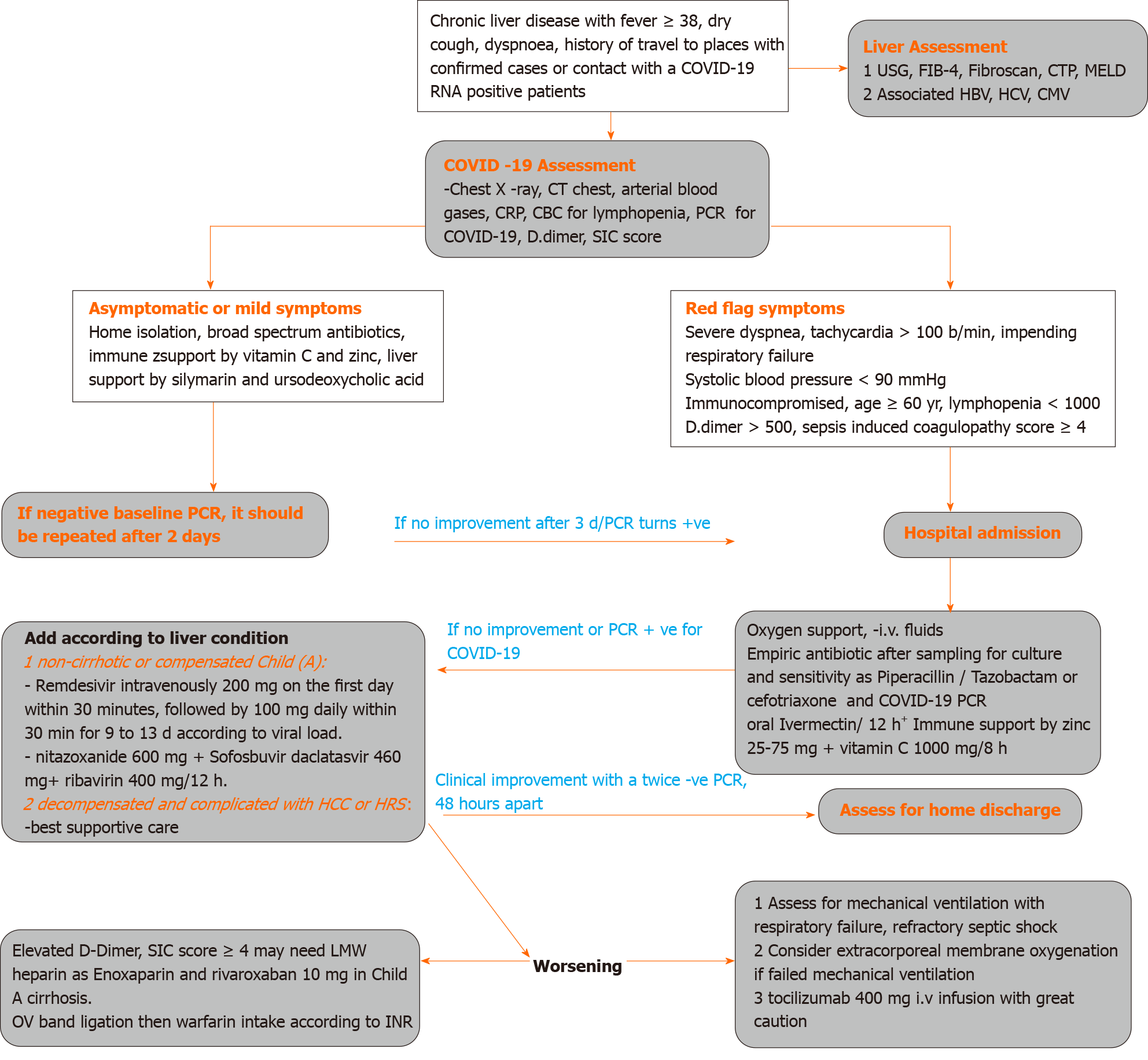



Challenges In Covid 19 Drug Treatment In Patients With Advanced Liver Diseases A Hepatology Perspective




Favipiravir A New And Emerging Antiviral Option In Covid 19 Sciencedirect


コメント
コメントを投稿